Dangsheng New Materials was selected as a member of the expert group of the first centralized technical unit for medical device packaging standardization

2024-03-15
Dangsheng New Materials was selected as a member of the expert group of the first centralized technical unit for medical device packaging standardization
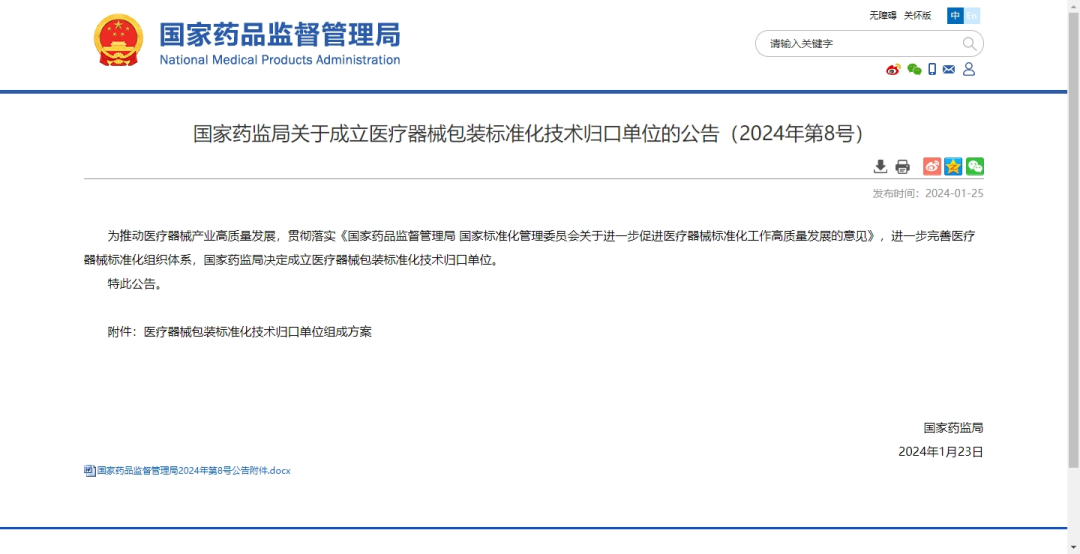
Standardization of medical device packaging Technical centralized unit In order to promote the high-quality development of the medical device industry, implement the opinions of the National Standardization Administration of the National Medical Products Administration on further promoting the high-quality development of medical device standardization work, and further improve the organizational system of medical device standardization, the National Medical Products Administration has decided to establish a technical centralized unit for medical device packaging standardization. On March 11th, the establishment meeting and the first meeting of the SMD/TU 011 Medical Device Packaging Standardization Technology Centralized Unit were held in Jinan. More than 60 officials from the China Institute for Food and Drug Control, Shandong Provincial Drug Administration, Shandong Medical Device and Drug Packaging Inspection and Research Institute, as well as all experts and industry representatives from the responsible units, attended the meeting. Dangsheng New Materials Medical Packaging Business Unit Technology Center Dr. Zhu Qianqin Selected as the centralized technical unit for standardization of medical device packaging List of expert groups The centralized unit for standardization technology of medical device packaging is mainly responsible for the formulation of general standards for packaging in the field of passive medical devices. This mainly includes standards such as terminology, guidelines, and methods for medical device packaging, as well as relevant standards for protective packaging of medical devices and sterile barrier systems. During his speech, Zhang Hui, the leader of the China Institute for Food and Drug Control (Center for Device Control), put forward requirements for the next steps of the responsible units: Firstly, strengthen the management of centralized units, establish standardized management systems, and ensure the healthy and steady development of centralized units; Secondly, strengthen expert management and strive to build and build a high-quality standardization work team; Thirdly, strengthen the process management of standard formulation and revision, focusing on the two key points of quality and efficiency; The fourth is to focus on building a standard system with a reasonable structure, advanced applicability, and international compatibility. List of personnel from centralized units The Secretariat is undertaken by Shandong Medical Device and Drug Packaging Inspection and Research Institute. The Medical Device Standards Management Center of the National Medical Products Administration is responsible for business guidance. The meeting discussed and approved the articles of association of the responsible unit, the work rules of the Secretariat, the work plan and standard system of the responsible unit. The conference invited industry experts to give a special report on the latest progress in medical device packaging research and organized training on standardization related knowledge. The attendees had sufficient communication and exchange with experts, and the atmosphere was lively.



Dawnsens News
Recommended news
-
Dangsheng New Materials was se
2024-03-15
-
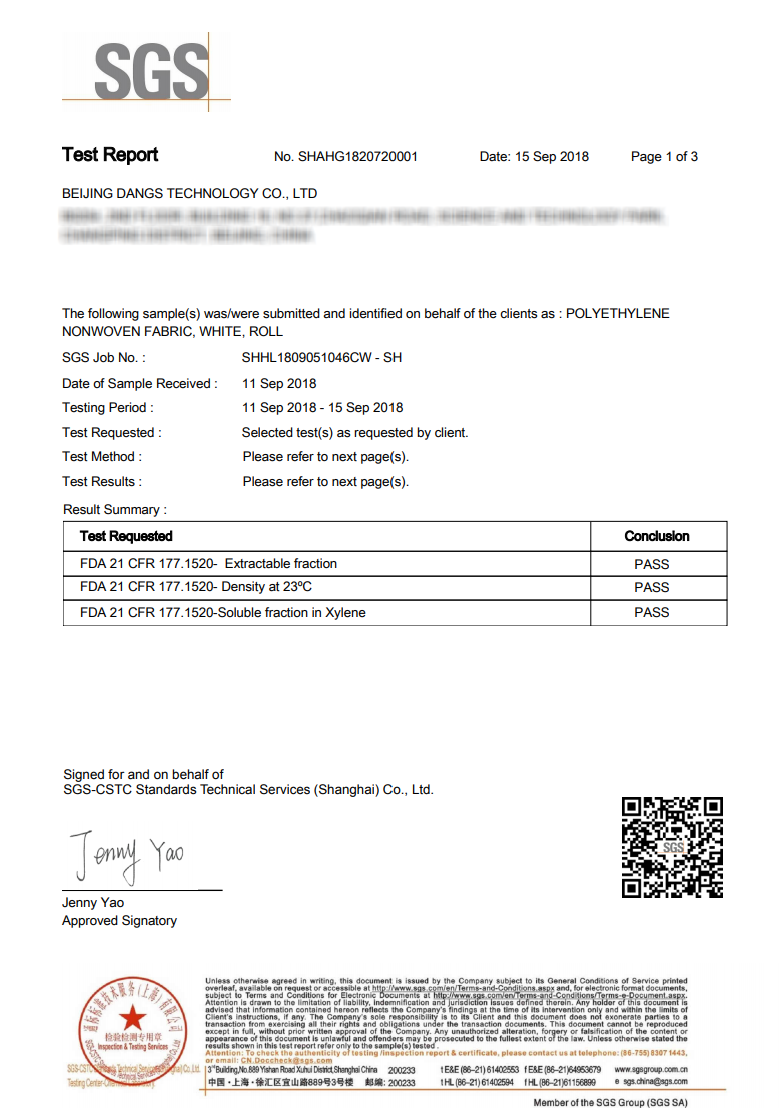
US Food Grade: U.S. FDA CFR 21
2024-06-27
-
REACH Substances of Very High
2024-06-27